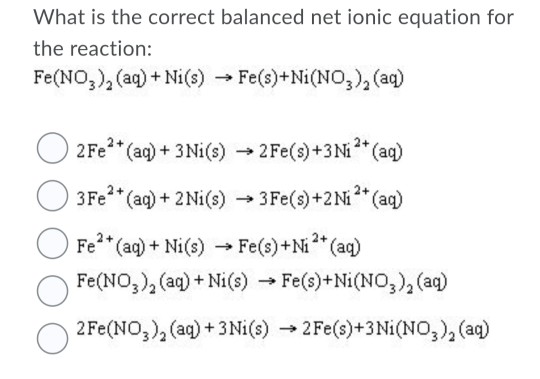Multicolour and reversibility of rewritable paper. a The reflective... | Download Scientific Diagram
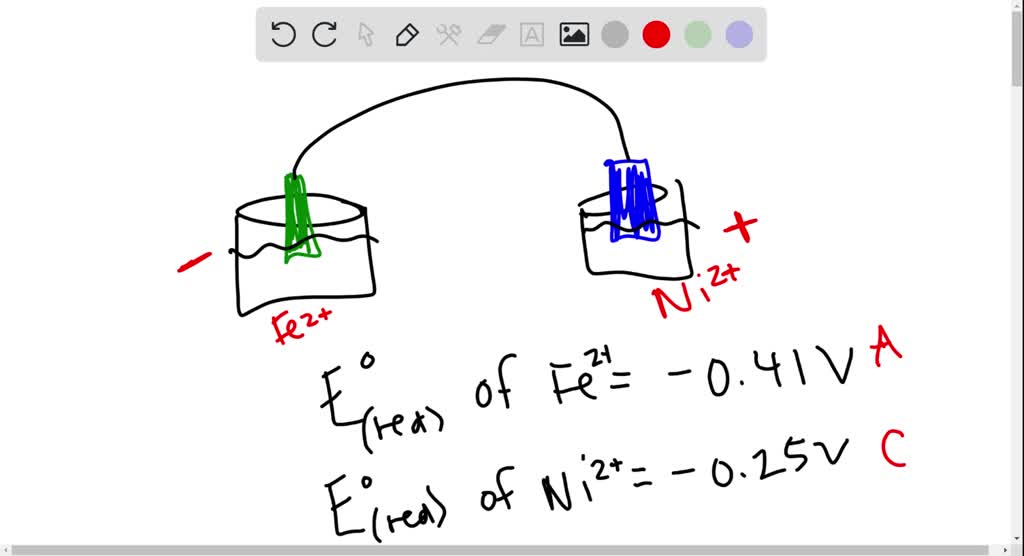
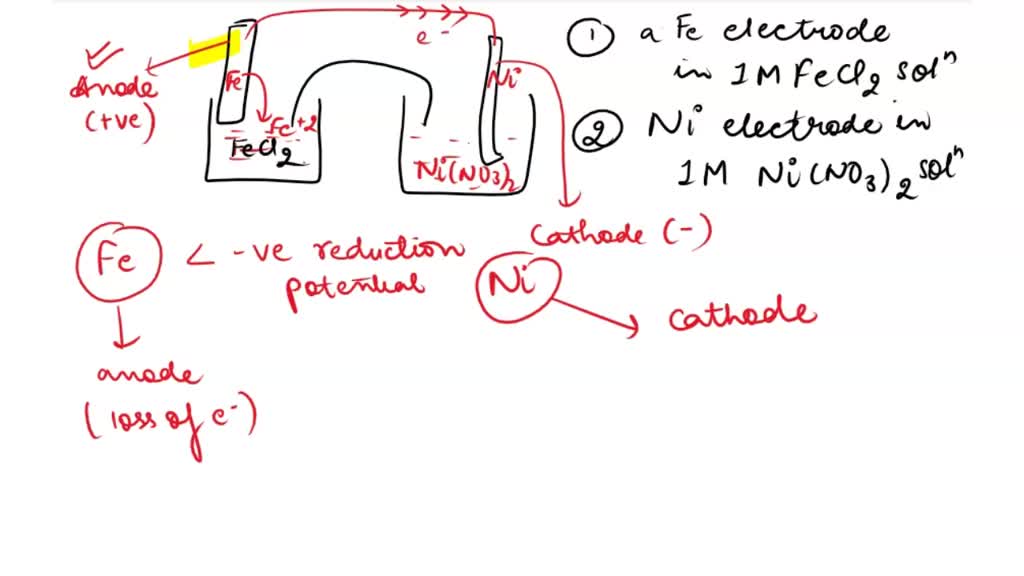
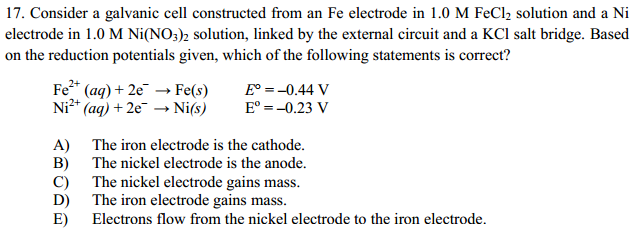
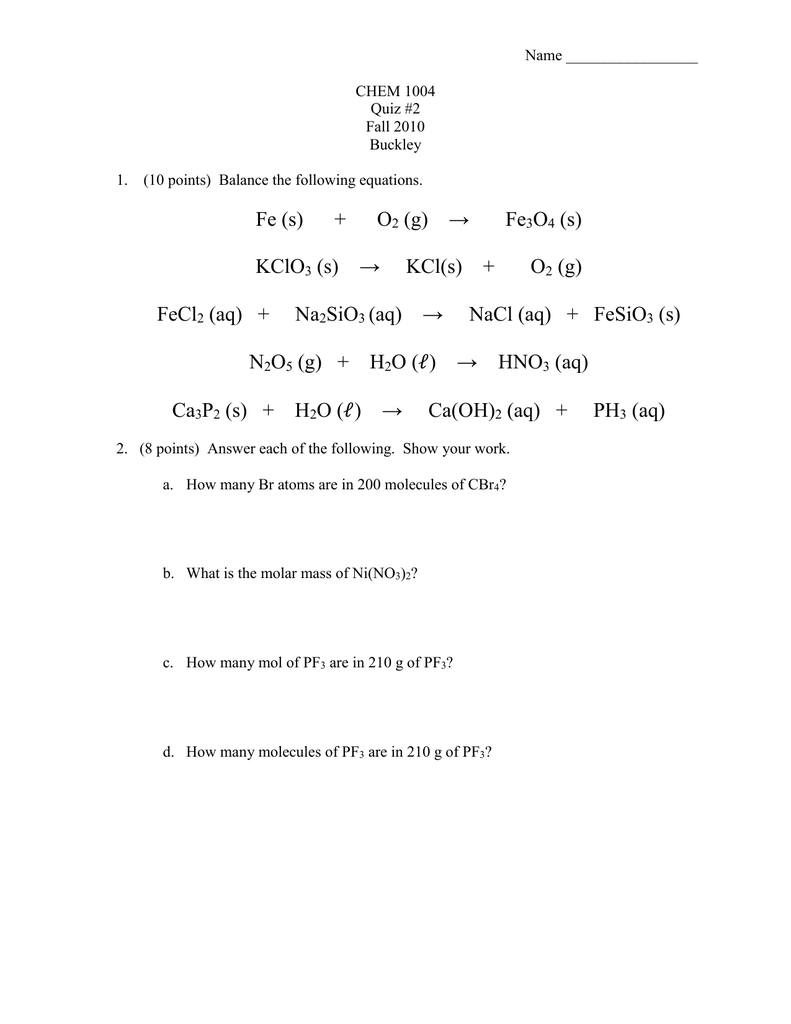
SOLVED: Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge: -a Fe electrode in 1.0 M FeCl2 solution -a Ni electrode in 1.0 M Ni(NO3)2
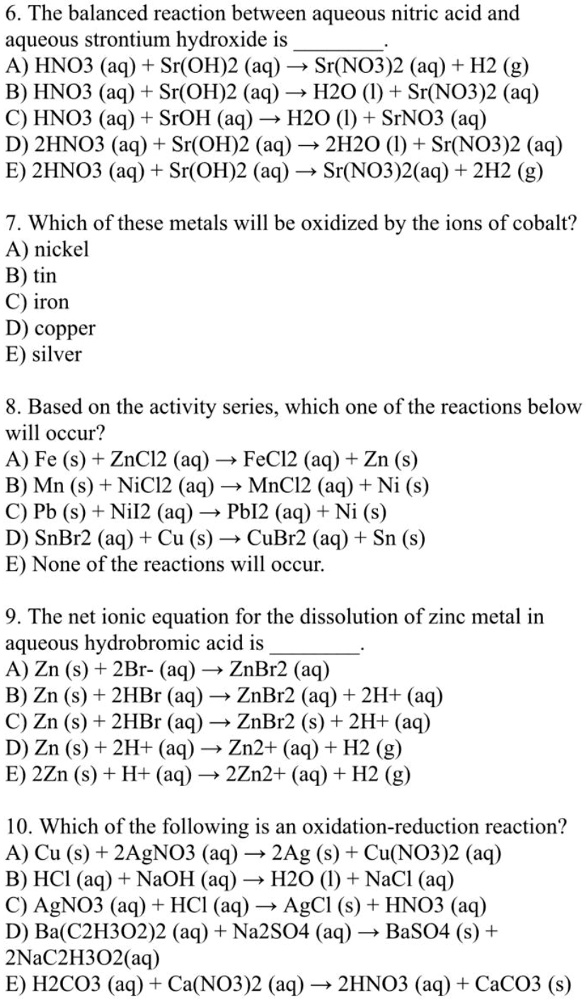
SOLVED: The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is HNO3 (aq) + Sr(OH)2 (aq) 57 Sr(NO3)2 (aq) + H2 (g) B) HNO3 (aq) + Sr(OH)2 (aq) 5 H2O (
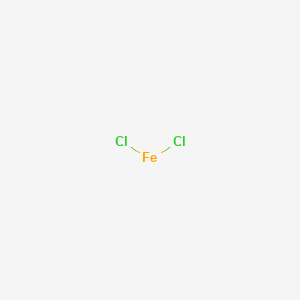
![PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a2e575c3a179f867fc9dc267d3158d61b9044f86/2-Figure1-1.png)
PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar

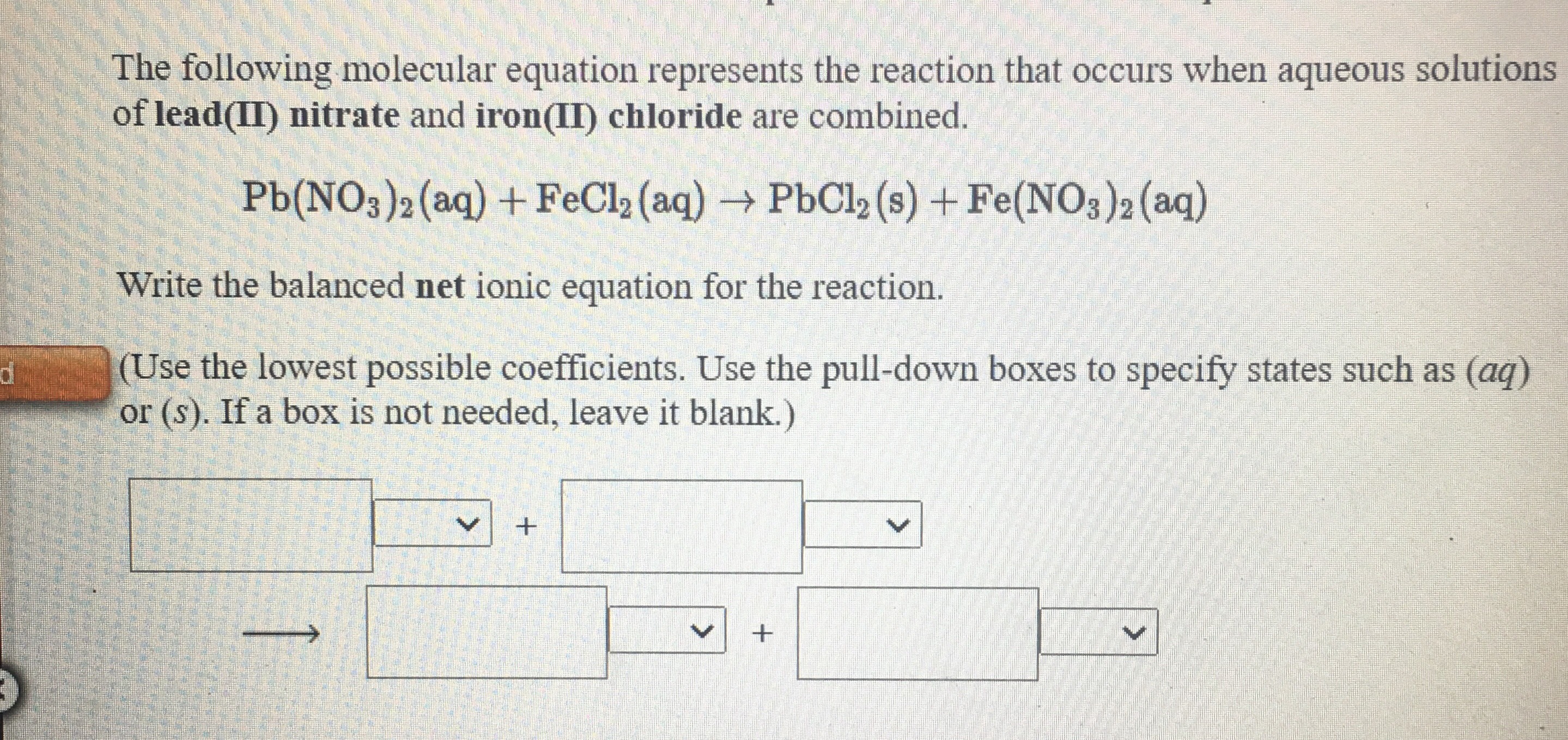
SOLVED: These compounds are in water: State the compound that forms a precipitate or indicate 'no precipitate' NaCl + Ba(OH)2 AgCIO3 Mgl2 CaCl2 K2CO3 FeCl2 (NH4)2S04 Ni(NO3)2 KOH Ni(NO3)2 K25 Pb(NO3)2 K2CO3
![PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a2e575c3a179f867fc9dc267d3158d61b9044f86/2-Table1-1.png)
PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar